- Home
- Industries
- Pharmaceutical
- Small Molecule
- Continuous Manufacturing

Integrated Monitoring and Control of Continuous Manufacturing
PCMM: Continuous Manufacturing
Reducing operating costs by up to 35%
Pfizer Inc has launched their Portable Continuous Miniature and Modular (PCMM) autonomous manufacturing unit for continuous oral solid dose form production. The equipment fits in a portable facility that can be shipped to any location, enabling medicines to be manufactured where and when they are needed. The advantages of PCMM are significant:
Reduced project timelines: installed within one week
Lower upfront investment costs, lower operating costs (up to 35% energy and resource savings)
Allows integration of a new vertical, in-line powder mixer for continuously blending pharmaceutical powder streams
Efficient experimental studies, estimated ~10 times less material usage, ~10 times faster than batch equipment
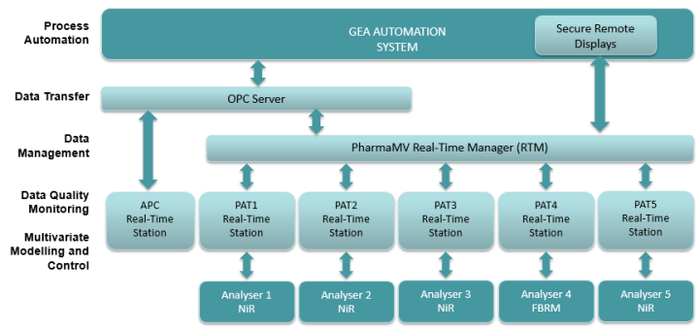
Allows up to five PAT devices within the continuous wet granulation and continuous direct compression process equipment
Integration of Perceptive's PharmaMV, to read process and PAT signals into a real-time monitoring and control system
Increased OEE and scale-up from R&D to full-scale commercial production using the same equipment
Both PAT and Advanced Process Control are seamlessly embedded into the operator interface. PAT is used to generate predictions of Critical Quality Attributes. Model Predictive Control adjusts key process actuations in a co-ordinated manner, to minimise variability in final product quality and respond to changes in raw material quality.
This provides a fully-integrated environment for process monitoring, product quality assessment and process control.