- Home
- Industries
- Pharmaceutical
- Pharma Solutions
- Continuous Process Verification

Continuous Process Verification
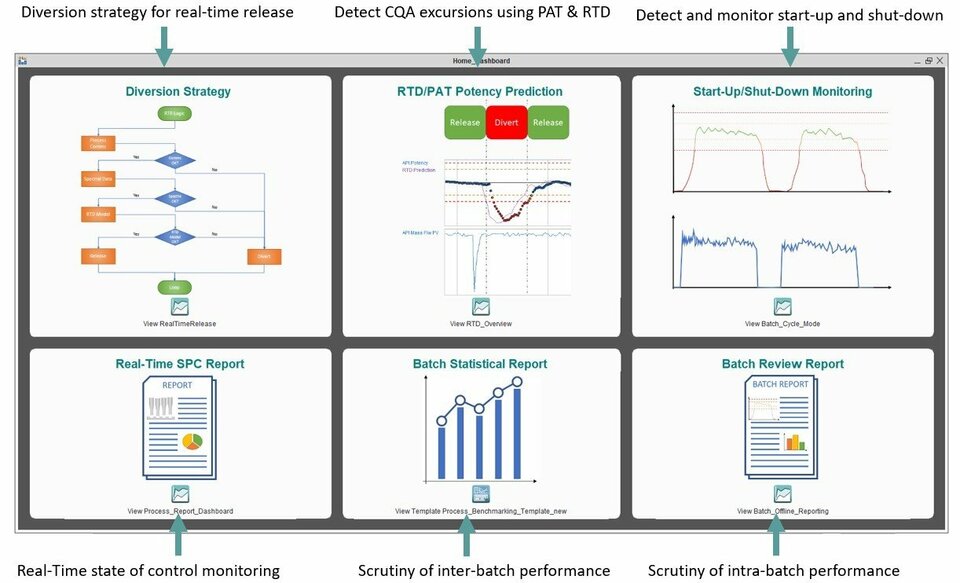
In pharmaceutical process and product development, design and qualification stages are succeeded by Continuous Process Verification (CPV). This provides ongoing assurance that the product attributes remain within compliance for regulatory bodies. PharmaMV provides a comprehensive set of CPV tools which facilitate the integration and interfacing to your process and production environment in order to:
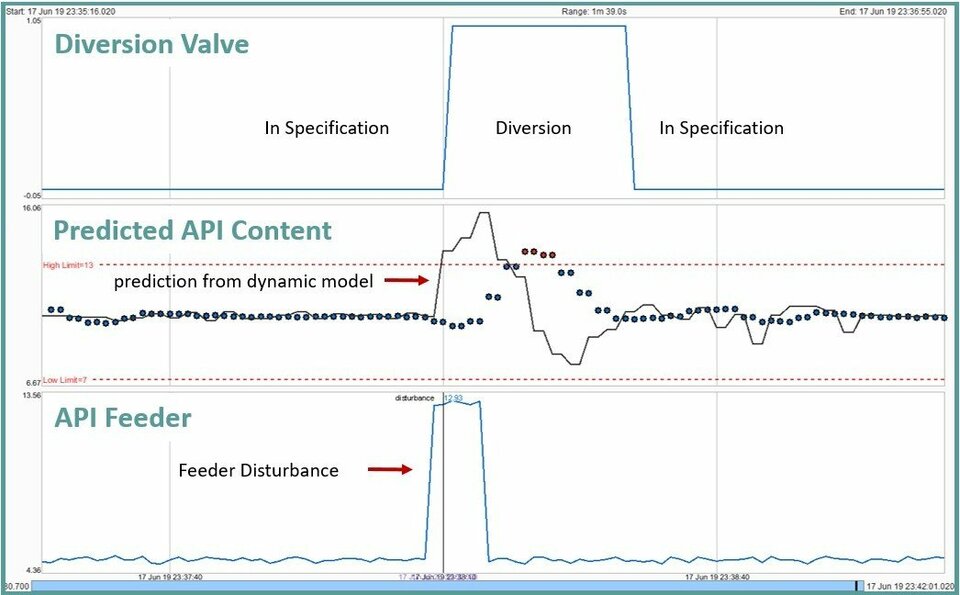
Report real-time results and soft-sensor data for critical quality attributes, CQA, prediction
Confirm that the process remains in a state of control during routine production
Comply with the FDA process validation
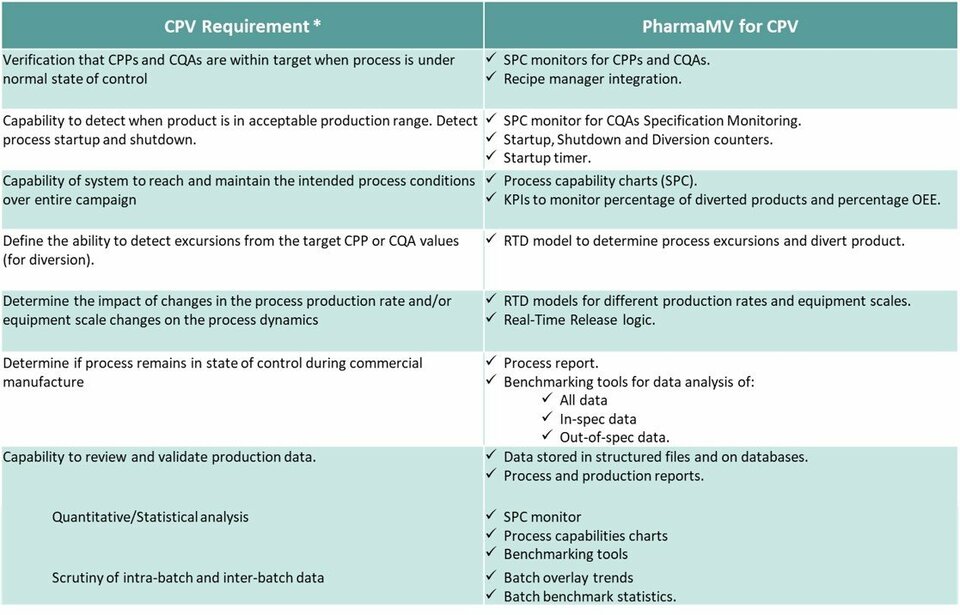
The CPV solution provides tools for the following requirements from FDA and EMA (ICH Q10) guidelines:
Verification of CPPs and CQAs when process is under normal state of control
Capabilities to detect when product acceptance criteria are met, including start-up and shutdown detection
Capability of verifying the system can reach and maintain intended process conditions over entire campaign
Capability of detecting excursions from target CPP and CQA values
Determining the impact of changes in production rate or equipment scaling on process dynamics
Capability to review and validate production data in quantitative/statistical analysis, including scrutiny of intra-batch and inter-batch data
*Continuous Manufacturing of Pharmaceuticals ISBN:9781119001324
How Can We Help?
If you want to discuss your project or process, please get in touch